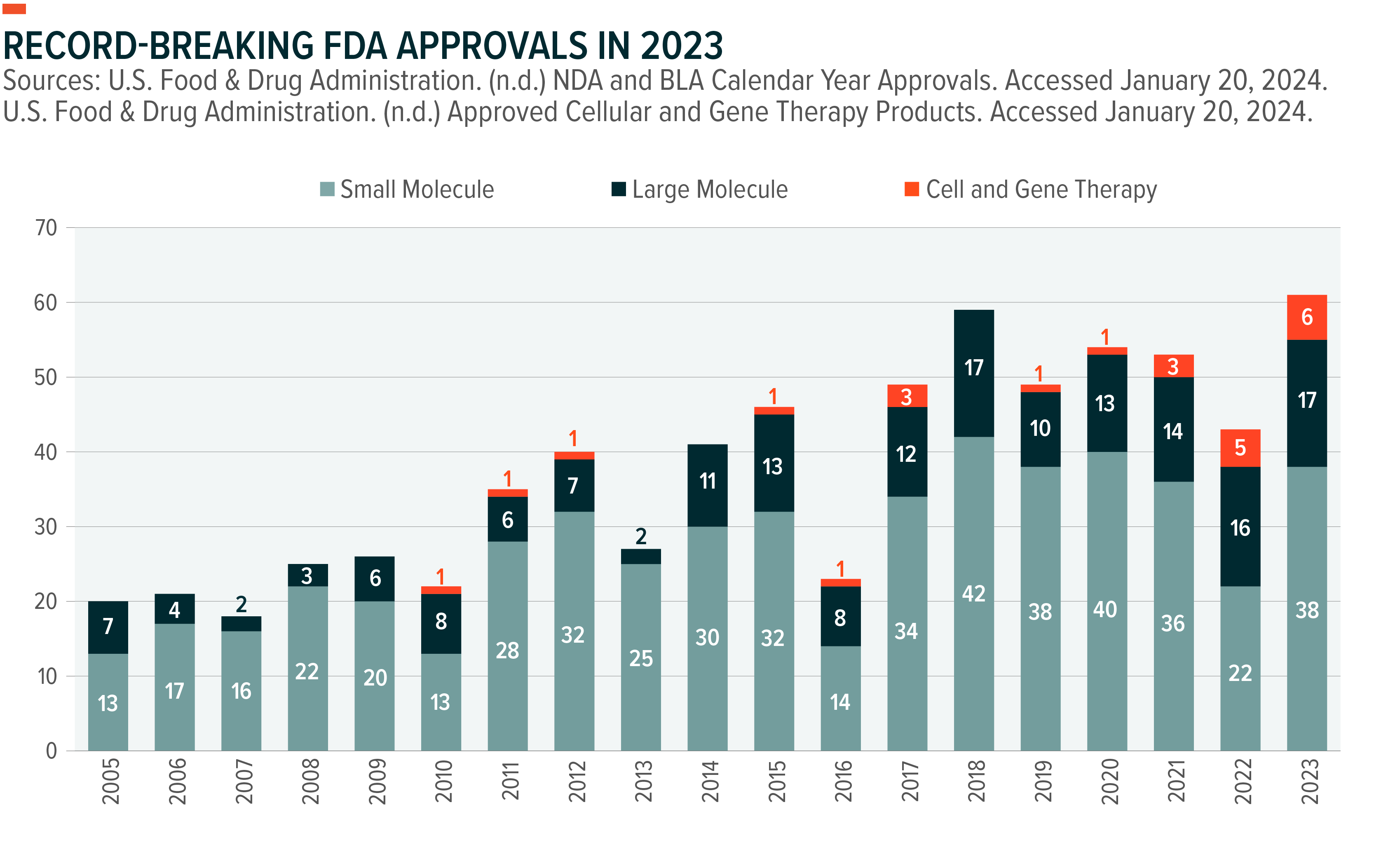
In 2023, the United States Food and Drug Administration (FDA) approved the highest number of new drugs in history (61), marking a record year in pharmaceutical development.1 This represented a 42% increase in approvals from 2022.2 Notably, the FDA awarded the first gene editing approval to CRISPR Therapeutics and Vertex Pharmaceuticals’ Casgevy.3 This was the sixth cell and gene therapy approval in 2023, bringing the total number of approved cell and gene therapies to 22.4
In last year’s iteration of this report, we highlighted the potential of three notable treatments, all of which were approved during 2023. These included Eli Lilly’s expanded approval of Mounjaro in obesity (approved as Zepbound), CRISPR Therapeutics’ gene editing treatment for sickle cell disease, and Sarepta Therapeutics’ gene therapy for Duchenne muscular dystrophy. These three treatments are expected to achieve combined 2028 sales of nearly $16 billion.5,6,7
In this piece, we look at 2024 and what could be more exciting developments in the biopharmaceutical market. We highlight three companies in the Global X Genomics & Biotechnology ETF (GNOM) and the Global X Aging Population ETF (AGNG) with noteworthy drugs up for approval.
Key Takeaways:
- A historical lack of success treating Alzheimer's, combined with a rapidly expanding patient population, could provide Eli Lilly with another exciting growth opportunity if its investigational drug Donanemab is approved.
- Moderna is looking to leverage its groundbreaking mRNA technology in applications beyond COVIID-19 vaccines, and it may find an answer in its RSV vaccine, mRNA-1345, which could be approved ahead of the 2024-2025 RSV season.
- Bristol Myers Squibb’s (BMS) growth-through-acquisition could see early benefits with KarXT, a treatment for schizophrenia developed by Karuna Therapeutics, which recently agreed to be purchased by BMS.
Eli Lilly’s Expected Approval in Alzheimer’s Bolsters Its Portfolio Growth
Eli Lilly’s story has been dominated recently by its blockbuster glucagon-like peptide 1 (GLP-1) treatments, Mounjaro and Zepbound. Beyond its success in type-2 diabetes and obesity management, the company is aiming to tackle the growing Alzheimer’s disease (AD) treatment space. An estimated 1 in 9 people aged 65 and older are affected by this debilitating condition.8 Eli Lilly currently awaits approval for its Alzheimer’s treatment, Donanemab, which, if approved, is expected to achieve peak annual sales of $2.5 billion.9
If approved, Donanemab could play an important role in Eli Lilly’s growth story. The company’s neurology sales are expected to increase at an 18% compound annual growth rate (CAGR) through 2028, the highest among all its segments.10
Industry wide, Alzheimer’s treatments are expected to grow at an estimated 57% CAGR through 2028, propelled by a growing patient population and historical lack of success in managing the illness. Currently, six million adults in the United States live with Alzheimer’s.11 By 2050, this number is expected to rise to nearly 13 million.12 The rapid aging of the global population is expected to drive the increase in Alzheimer’s cases through 2100.
Donanemab: An Expected Blockbuster Drug in Alzheimer’s Disease
Alzheimer’s disease has historically been a difficult illness to treat and prevent given its complex nature and different presentations across patients. Despite decades of dedicated research, we’ve seen little success in investigational treatments slowing disease progression. Eli Lilly seeks to change the prognosis for Alzheimer’s patients with its investigational Donanemab treatment.
Donanemab targets accumulation of amyloid beta protein in the brain. The belief is that, when treated early, a decrease in amyloid beta accumulation can lower the risk of disease progression.13 In its phase III clinical study, Donanemab reduced amyloid plaque on average by 84% at 18 months, compared to a 1% decrease for patients on placebo.14 The trial also showed Donanemab slowed cognitive decline by 35% and showed 39% lower risk of progressing to the next stage of the disease when compared to placebo.15 The data are particularly encouraging, given that Biogen and Eisai’s Leqembi, approved in 2023 for Alzheimer’s, slowed cognitive decline by 27%.16
Eli Lilly: Leveraging Diagnostic Capabilities to Drive Treatment Adoption
Eli Lilly could receive approval for Donanemab as early as the first quarter of 2024. If approved, Eli Lilly is projected to capture 25% of the $11.3 billion Alzheimer’s treatment market in 2028, which would make it the second-highest grossing company in the Alzheimer’s treatment space.17
The firm is also looking to improve diagnostic capabilities for Alzheimer’s. Currently, patients are diagnosed, on average, 2.8 years after symptom onset, and 75% of people living with the symptoms of Alzheimer’s are undiagnosed.18 The recent success in slowing cognitive and functional decline in Alzheimer’s patients has been largely concentrated in early-stage cases, highlighting the need for early detection.
To better support patients with Alzheimer’s early in their disease progression, Eli Lilly recently partnered with Roche on the development of a novel blood test to facilitate the early diagnosis of Alzheimer’s.19 If approved, the test could determine whether patients should receive further evaluation that may confirm a diagnosis and could help them access new therapies as they become available. The Alzheimer’s diagnostics market, on its own, is projected to be an $8 billion opportunity.20
Moderna Expects to Validate mRNA Technology Beyond COVID-19
Messenger RNA (mRNA) firm Moderna expects its first launch of a non-COVID-19 related vaccine in 2024. The company has spent the last few years expanding its investigational efforts, aided by the $45.8 billion it has brought in with its COVID-19 vaccine, Spikevax.21 Moderna now has 45 development programs, up from 25 in 2020.22 Fueled by its recent efforts, its investigational vaccine for respiratory syncytial virus (RSV), mRNA-1345, is expected to receive FDA approval in 2024.23
RSV is a seasonal, highly contagious respiratory virus that often feels like a common cold but can be dangerous among infants, young children, and senior citizens. The RSV market is expected to be worth over $10 billion by 2030, up from $900 million in 2022, fueled by increasing hospitalizations for RSV patients and new technological advancements to prevent the illness.24
The approval of mRNA-1345 could mark the first of four expected novel approvals through 2025 that would likely allow Moderna to return to growth after the decline in COVID-19 revenue.25 The firm also has high hopes for mRNA technology beyond infectious diseases, including its investigational vaccines for melanoma and lung cancer.
Leveraging Expertise in Infectious Diseases to Help Prevent RSV
In the U.S., an estimated 60,000-120,000 older adults are hospitalized annually, and 6,000 to 10,000 of them die, due to RSV infection.26 Moderna hopes to receive approval for mRNA-1345 in adults in the first half of 2024, ahead of the 2024-2025 RSV season.27 If approved, mRNA-1345 would compete against two recently approved vaccines. GSK’s Arexvy, the first FDA-approved RSV vaccine, was approved in May 2023 and achieved sales of $1.5 billion in just four months since its launch.28 Pfizer’s Abrysvo, for its part, received approval in August 2023 and achieved $515 million in sales since its launch.29 Neither leverage mRNA technology.
Thus far, Arexvy has largely dominated the nascent RSV market, though Moderna believes it can compete. In the ConquerRSV trial, mRNA-1345 was found to be 83.7% effective against RSV, giving it an edge over both Arexvy and Abrysvo, which have reported to be 82.6% and 61.1% effective, respectively.30
Moderna is also running clinical trials to receive subsequent pediatric and maternal approvals for mRNA-1345, which combined represent around 10-20% of the total RSV opportunity.31
Laying the Groundwork for a Comprehensive Infectious Disease Portfolio
Moderna’s potential approval in RSV is expected to help set up the firm for a host of infectious disease vaccine approvals, including a single mRNA vaccine that can combine COVID-19, influenza, and RSV shots. Combination vaccines would provide notable differentiation from traditional immunization methods, helping scale manufacturing efforts and facilitate higher immunization rates across seasonal infectious illnesses. mRNA technology is particularly well-suited for combination immunization compared to traditional vaccine methods.32
Moderna’s therapy development process allows for rapid deployment of vaccines with projected 60-day timelines prior to in-human clinical trials.33 The firm also reports having a greater average success rate with clinical trials than the industry average – nearly double the industry average for phase I trials and three times the industry average for phase II trials.34
Bristol Myers Squibb Bets on Growing Neuroscience Market
Karuna Therapeutics, which recently agreed to be acquired by Bristol Myers Squibb for $14 billion (pending regulatory approval), currently awaits approval for its investigational schizophrenia treatment, KarXT.35 If given the green light, the treatment would represent the first novel pharmacological approach to treating schizophrenia in several decades.36 KarXT is expected to achieve 2028 sales of $2 billion and could reach peak sales of $6 billion.37,38
Bristol Myers Squibb has played a limited role in the neuroscience market in recent years, with oncology, musculoskeletal, and oncology treatments bringing in the majority of its annual revenues.39 Given recent loss of exclusivity for key revenue generating therapies, Bristol Myers Squibb has focused on driving growth via recent drug launches and bolt-on acquisitions. The firm has highlighted 16 potential growth catalysts in 2024, including the expanded approval of its CAR-T treatment, Abecma, in less pre-treated patients and the approval of KarXT in schizophrenia.40 The Karuna acquisition is expected to close in the first half of 2024, while Bristol Myers prepares to support the launch of KarXT.41
KarXT: A Novel Approach for an Underserved Population
If approved, Karuna and Bristol Myers Squibb could launch KarXT in the second half of the year.42 The drug targets both M1 and M4 muscarinic receptors, which play a key role in various psychiatric and neurological disorders. By targeting both receptors, KarXT can help reduce symptoms of psychosis and counter potential side effects. This notably differs from the current methods of treating schizophrenia and other similar disorders, which solely rely on blocking dopamine or serotonin receptors.
The revolutionary treatment stands to benefit the 24 million individuals with schizophrenia worldwide.43 The illness has historically been underserved and the few treatments on the market usually come with notable side effects like weight gain and drowsiness. Only an estimated 20% of schizophrenia patients are considered “well treated.”44
Bristol Myers Squibb’s Opportunity in Neuroscience
Against this backdrop, KarXT offers a strong efficacy profile with improved side effects compared to existing treatments. In a phase III clinical trial, KarXT showed an 8.4-point reduction compared to placebo in the Positive and Negative Syndrome Scale (PANSS), the industry standard for measuring the severity of schizophrenia symptoms.45 The trial also showed KarXT triggered a 3.5-point benefit in the reduction of “positive” schizophrenia symptoms like hallucinations and delusions.46
KarXT’s success in schizophrenia also opens the door for the treatment of other neurological disorders. KarXT is already being investigated in Alzheimer’s-related psychosis and is believed to be a potential good fit for bipolar disease.47 Via the acquisition of Karuna, Bristol Myers Squibb would also gain access to Karuna’s investigational treatments for other neurological disorders like anxiety.
Conclusion
With potential approvals forthcoming, we believe Eli Lilly, Moderna, and Bristol Myers Squibb can help pave the way towards a more effective patient treatment paradigm across key disease areas. For investors, the potential approvals of Donanemab, mRNA-1345, and KarXT could be notable growth catalysts, given their large addressable markets and a historical lack of success in addressing Alzheimer's, RSV, and schizophrenia. With exposure to the Genomics & Biotech and Aging Population investment themes, investors can capture breakthroughs like these and potentially benefit from their growth as they disrupt traditional patient care for the better over time.
Related ETFs
GNOM – Global X Genomics & Biotechnology ETF
AGNG – Global X Aging Population ETF
Click the fund name above to view current holdings. Holdings are subject to change. Current and future holdings are subject to risk.